
Exercise-Induced Rhabdomyolysis
CITATION:
Senert R, Kohl L, Rainone T, Scalea T: Exercise-induced rhabdomyolysis.
Ann Emerg Med June 1994;23:1301-6.
AUTHORS:
Richard Sinert, DO  Lewis Kohl, DO
Lewis Kohl, DO
Teresa Rainone, MD  Thomas Scalea, MD
Thomas Scalea, MD
ABSTRACT:
Study objective: To describe the syndrome of exercise-induced rhabdomyolysis and to investigate the relation between exercise-induced rhabdomyolysis and the development of acute renal failure.
Design: Retrospective chart analysis on all patients with a discharge diagnosis of rhabdomyolysis from January 1988 to January 1993.
Setting: An urban tertiary care center with 225,000 annual emergency department visits.
Type of participants: Thirty-five patients met the inclusion criteria for exercise-induced rhabdomyolysis: a history of strenuous exercise, creatine phosphokinase level more than 500, and urine dipstick positive for blood without hematuria. We excluded patients with a history of trauma, myocardial infarction, stroke, or documented sepsis. Charts also were examined for the presence of nephrotoxic cofactors (ie, hypovolemia and/or acidosis).
Results: All 35 patients were men without significant past medical history and were an average age of 24.4 years. The average admission creatine phosphokinase was 40,471 U/L. No patient presented with or developed nephrotoxic cofactors during hospitalization. None of our study patients experienced acute renal failure.
Conclusion: Previous literature has described a 17% to 40% incidence of acute renal failure in rhabdomyolysis. None of our patients developed acute renal failure, signifying a much lower incidence of acute renal failure in exercise-induced rhabdomyolysis without nephrotoxic cofactors than in other forms of rhabdomyolysis.
Exercise-Induced Rhabdomyolysis
INTRODUCTION
Since the original description of the syndrome of muscle pain, weakness, and brown urine by Meyer-Betz in 1910,(1) numerous case reports have linked rhabdomyolysis to such strenuous activities as military basic training(2,3) and weight lifting.(4) Knochel(5) has termed exercise-induced rhabdomyolysis "white collar rhabdomyolysis" because of its high incidence in intelligent, well-educated professionals who can arrange their work schedules to allow for daily running. Although training probably decreases the risk of rhabdomyolysis, this syndrome also has been reported commonly in professional athletes during marathon races(6) and ice skating competitions.(7)
Rhabdomyolysis appears to be a relatively common sequela of strenuous exercise. In the largest screening to date, Olerud et al(8) sampled blood for myoglobin in 337 military recruits during their first six days of conditioning and found approximately 40% to have some degree of rhabdomyolysis.
In the classic description,(5) these patients after strenuous exercise will present initially with confusion, pallor, and hyperthermia. These symptoms improve rapidly with rehydration, only to have the patient rapidly deteriorate in the next 12 to 24 hours with acute renal failure, hyperkalemia, and disseminated intravascular coagulation.
This clinical scenario was first termed "crush syndrome" by Bywaters and Beall(9) in 1940. During the London Blitz, they observed multiple patients whose limbs were crushed and who survived the initial shock state only to die days later of uremia. Bywaters and Stead later identified the toxin responsible for acute renal failure by reproducing the syndrome in rabbits with the injection of myoglobin.(10)
In murine models of acute renal failure, myoglobin injections without coexistent hypovolemia and/or aciduria (nephrotoxic cofactors) rarely caused decreases in glomerular filtration rate.(11,12) A medical literature review of all case reports of human myoglobinuric acute renal failure shows a consistent presentation complicated by these nephrotoxic cofactors. We describe in this report our clinical experience with 35 cases of exercise-induced rhabdomyolysis with evidence of significant myoglobinemia (demonstrated as markedly elevated creatine phosphokinase [CPK]) without complicating nephrotoxic cofactors, a syndrome that may represent a "pure" form of rhabdomyolysis.

MATERIALS AND METHODS
This study was designed as a retrospective cohort analysis of all cases of exercise-induced rhabdomyolysis. Medical records were reviewed for a five-year period (January 1988 to January 1993) using a computer search of the ICD-9 code (728.89) for both the primary and secondary discharge diagnoses of rhabdomyolysis. The study inclusion criteria for exercise-induced rhabdomyolysis were an elevated CPK concentration of more than 500 U/L (normal, 150 U/L or less), myoglobinuria, and a history of heavy exertion. Myoglobinuria was defined by a urine test (orthotoluidine) positive for blood without gross hematuria (RBC count more than 5 per high-power field [hpf]). None of our patients presented with hemolysis or other conditions that would have increased urinary levels of hemoglobin or bilirubin, which also produce a positive orthotoluidine test. Patients were excluded if any evidence of trauma, myocardial infarction, or stroke could be found by chart analysis. Initially, 75 cases of rhabdomyolysis from all causes were examined. Thirty-five cases met the inclusion criteria for exercise-induced rhabdomyolysis and are reviewed in this report. All charts were reviewed by the authors.
Initially, patients were evaluated and diagnosed and had treatment started in the emergency department. All patients had blood drawn within 24 hours of admission for sodium, potassium, chloride, bicarbonate, creatinine, calcium, and CPK. An initial urine analysis was performed on all patients on the day of admission. Blood and urine tests for toxicology (cocaine, barbiturates, opiates, LSD, alcohol, PCP) were sent within 24 hours of admission.
All patients were admitted to Kings County Hospital Center. Serial laboratory tests were obtained in every patient throughout their admission. Average values are presented with standard deviations.

RESULTS
The study population consisted of 35 male patients with an average age of 24.4 ± 5.4 years. Nineteen patients were black, 15 were Hispanic, and one was white. All patients were imprisoned at Rykers Correctional Institution at the time of admission.
Past medical history did not disclose previous episodes of rhabdomyolysis in any of the patients. No patient described similar histories of muscle weakness from exercise or family members with any muscle problems. Although nine patients gave a history of ethanol abuse, no patient admitted to intake within 24 hours before heavy exercise. Eight patients had a history of drug abuse, but all denied use at the time of exertion. None of the patients admitted to taking nonsteroidal anti-inflammatory drugs within 24 hours of muscle pain from exercise. Urine and blood toxicologic tests were all negative for all patients.
Thirty-one of the patients did squat-thrusts with or without weights, two patients did chin-ups, one patient lifted heavy weights, and one ran a long distance. These repetitive exercises were done not as part of a usual exercise routine but as a consequence of losing a game of dominoes. The number of squat-thrusts varied between 75 and 900, with weights from 40 to 170 lb.
Patients presented an average 2.7 ± 0.3 days (range, 0 to 5 days) after exertion. All patients complained of thigh or arm pain. A change in urine color was noted by every patient. On initial vital signs, no patient was found to be hypotensive (systolic blood pressure less than 100 mm Hg), tachycardic (pulse more than 100), or orthostatic (drop in systolic blood pressure more than 10 mm Hg or pulse change more than 10 with change in position) to any significant degree of hypovolemia. No patient presented with frank hyperthermia (temperature more than 39.4° C), flushing, or lack of sweating characteristic of heat stroke. On physical examination, trace edema of the involved extremities was the only consistent finding.
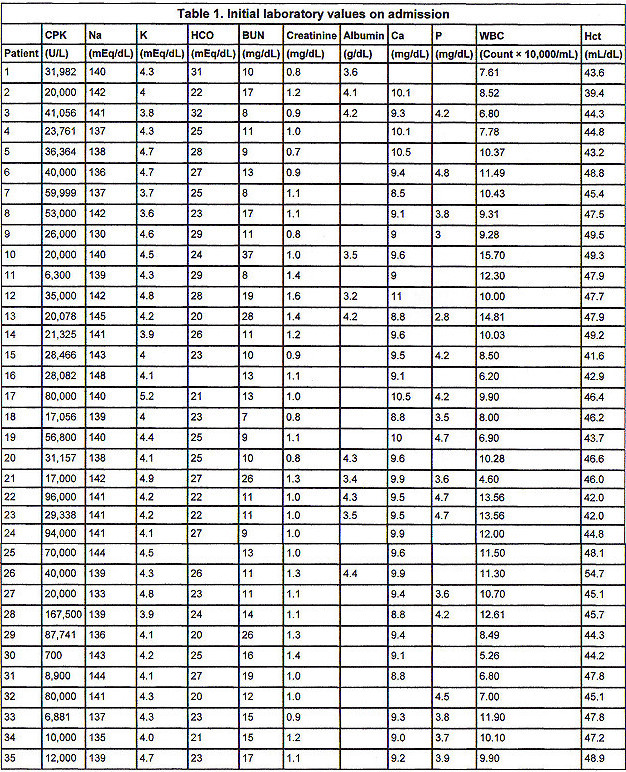
From the initial laboratory values in Table 1, the CPK on admission was 40,471 ± 34,295 U/L (range, 700 to 165,000 U/L). No patient presented with hyperkalemia, acidosis, hypocalcemia, or hyperphosphatemia. No patient presented with elevated creatinine (more than 2.0 mg/dL). or elevated BUN (more than 25 mg/dL). Serum albumin, obtained in only 11 patients, was 3.88 ± 0.44 g/dL. Hemoglobin and hematocrit did not reveal anemia or hemoconcentration in any patient. No patient presented with thrombocytopenia or had evidence of disseminated intravascular coagulation.
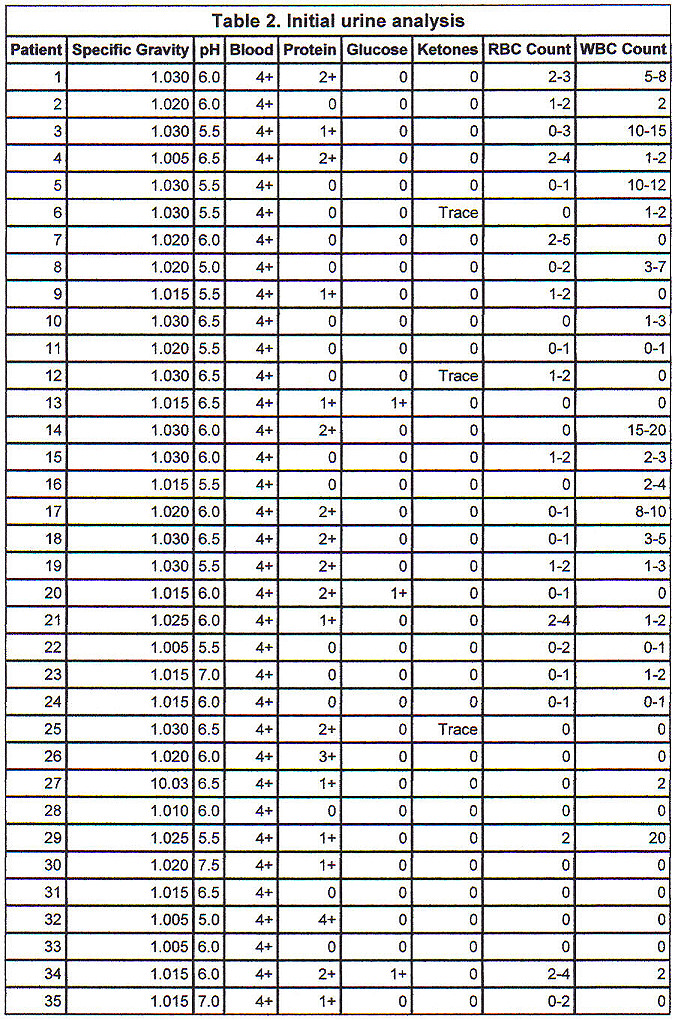
Initial urine analysis in Table 2 shows all patients to have had 4+ orthotoluidine reaction (urine dipstick) without microscopic hematuria (more than 5 RBCs/hpf). No patient had significant glycosuria or ketonuria. The average specific gravity was 1.020 ± 0.01. Average urine pH was 6.03 ± 0.55 pH units. The presence of protein and WBCs in the urine was variable.
The average patient's hospitalization was 6.73 ± 1.03 days. All patients except patient 6 were treated with forced bicarbonate diuresis. In addition, two patients, patients 9 and 12, received a mannitol infusion on admission. These two patients received mannitol infusions by the attending physician's preference; no clinical or laboratory criteria unique to these patients could be identified. Laboratory values were obtained during their hospital stay, and no patient developed hyperkalemia, acidosis, hypocalcemia, or hyperphosphatemia. No patient experienced an elevation of BUN or creatinine above initial values or required dialytic therapy on an acute or chronic basis after this illness.

DISCUSSION
The clinical significance of rhabdomyolysis lies in its association with myoglobinuric acute renal failure and its subsequent mineral and electrolyte derangements. The 35 cases reported in this study are unique not only for their similarity in presentation but also for their lack of acute renal failure.
The incidence of acute renal failure secondary to rhabdomyolysis from all causes has been investigated. Studies by Ward,(13) Gabow et al,(14) and Kageyama(15) found the incidence of rhabdomyolysis-induced acute renal failure to be 17%, 33%, and 35%, respectively. Another study by Akmal et al(16) described a 40% incidence of acute renal failure from rhabdomyolysis caused by phencyclidine. What, then, explains the lack of acute renal failure in our cohort?
Our patients clearly had very significant rhabdomyolysis, as evidenced by their markedly elevated CPKs and positive urine dipstick for blood (without hematuria). CPK has been demonstrated previously as a marker for muscle damage. Studies that have measured simultaneous serum CPK and urine myoglobin show a consistent relation.(12,17) Interestingly, these studies found no such relation between CPK levels and the incidence of acute renal failure. Our patient's CPKs were on average 40,471 ± 34,295 U/L on admission, levels similar to those in other studies in which acute renal failure was common.(5,6,16,18) In addition, although myoglobin was not measured directly in this study, a positive urine dipstick for blood requires at least 100 mg/dL of myoglobin or hemoglobin to be present in the urine sample.(19) This test was positive in all of our study patients.
Medical intervention also was considered as an explanation for our patients' lack of acute renal failure, because 90% received forced bicarbonate diuresis. In the majority of cases (31 of 35), treatment was delayed more than 48 hours after exercise because of late presentations for medical care. Although no human controlled studies are available defining the optimal time for initiating forced bicarbonate diuresis, studies by Better and Sterin(20) and Ron et al(21) suggest an upper time limit for effectiveness. The authors describe two groups of patients with similar degrees of rhabdomyolysis. One group treated with bicarbonate diuresis within six hours of injury had no patients with acute renal failure. In contrast, the group with a greater-than-six-hour delay in starting bicarbonate all developed acute renal failure. We believe that any therapies received by our patients probably were too late to effect a favorable outcome, and we suggest other possibilities for our patients' lack of acute renal failure.
Retrospective reviews by Ward(13) and Gabow et al(14) have studied the predictors of acute renal failure in a broad range of etiologies of rhabdomyolysis. These reviews using multivariate analysis have produced formulas based on initial laboratory values that were highly predictive of acute renal failure in their populations. Neither study to date has been validated in a prospective manner. Interestingly, acute renal failure was associated with a normal range of albumin in Gabow et al's(14) study, and a low albumin was statistically significant for acute renal failure in the study by Ward.(13) This disparity probably results from differences in study populations resulting from dissimilar mixes in the etiologies of rhabdomyolysis. In our study, only 11 of 35 patients had a documented serum albumin, preventing the authors from testing these two formulas for predicting acute renal failure in our population.
Our retrospective cohort study suggests that the absence of nephrotoxic cofactors (signficant hypovolemia and/or aciduria) in the setting of exercise-induced rhabdomyolysis explains our patients' lack of acute renal failure. In reviews(5,13-17,21,21) of myoglobinuric acute renal failure, a consistent finding of these nephrotoxic cofactors was noted. This appears to implicate renal ischemia and perhaps acidosis/aciduria as necessary cofactors in the development of myoglobinuric acute renal failure.
Murine models of myoglobinuric renal failure have demonstrated myoglobin as relatively nonnephrotoxic.(11,12,23) IV injections of hemoglobin/myoglobin have failed consistently to produce decreases in glomerular filtration rate unless the animals were first made hypovolemic.(24,25)
The nephrotoxic role of aciduria in myoglobinemia also has been experimentally elucidated. In the presence of acidic urine (pH below 5.6), myoglobin dissociates into globulin and hematin.(26) Infusions of hematin have been shown to cause more consistent decreases in glomerular filtration rate than does myoglobin.(27) The toxic effect of hematin on a cellular level has been ascribed to the heme iron production of free hydroxy radicals.(28) In urine below pH 5.0, the solubility of myoglobin decreases dramatically. In this pH range, myoglobin cast formation and the percentage of myoglobin retained in tubules increase, which has a high correlation with the development of acute renal failure.(11)
None of our patients developed hypocalcemia or hyperkalemia, both of which are associated with rhabdomyolysis. Whether these complications were not observed because of the absence of acute renal failure could not be determined but seems likely.
In trying to apply the results from our very homogeneous group (age, sex, race, and type of exercise) to the more general case of "white collar rhabdomyolysis," several limitations must be acknowledged. Our retrospective study design by its nature could not guarantee that all patients who would have met the inclusion criteria were found by searching for the ICD-9 code (728.89) for rhabdomyolysis. The ability to collect a consistent data set also was limited in our study by its design, especially in obtaining serum albumin in all patients. In addition, the absence of any cases of myoglobinuric acute renal failure does not allow for the converse conclusion that the presence of nephrotoxic cofactors are both necessary and sufficient for acute renal failure in these patients.

CONCLUSION
Rhabdomyolysis is a relatively common complication of strenuous exercise, as evidenced by the military recruit data(8) and the large number of reports of "white collar rhabdomyolysis" gathered by Knochel.(5) Reports of exercise-induced rhabdomyolysis in professional athletes(6,7) support our experience that neither the amount of exercise nor the level of training appears to be a reliable predictor for the development of rhabdomyolysis.
Exercise-induced rhabdomyolysis accounted for 47% of our admissions for rhabdomyolysis but was not responsible for a single case of acute renal failure. With reported incidences of acute renal failure in rhabdomyolysis ranging from 17%(13) to 40%,(16) we would have expected to have seen between six and 14 patients with acute renal failure in our sample of 35 patients. From our data and a review of the clinical literature, exercise-induced rhabdomyolysis without complicating nephrotoxic cofactors has a significantly lower incidence of acute renal failure than other forms of rhabdomyolysis. It is doubtful that the varying etiologies of rhabdomyolysis alone would affect the incidence of acute renal failure. Instead, the importance of nephrotoxic cofactors in the development of myoglobinuric acute renal failure must be considered. This hypothesis is supported by a review of murine models in which hypovolemia and/or aciduria were found to be necessary for the development of acute renal failure.
Although a role for forced bicarbonate infusion has been defined for trauma patients(20,21) in preventing myoglobinuric renal failure, our study did not address its use in exercise-induced rhabdomyolysis. Although our data may suggest that acute renal failure occurs rarely if at all in these patients, hospital admission still seems prudent to prevent dehydration during their period of immobility from muscle injury.

REFERENCES

Publishing and Reprint Information

 Email Charly at: c-d-miller@neb.rr.com
Email Charly at: c-d-miller@neb.rr.com