 |
 |
of Asphyxia in Lungs.
A Semiquantitative Study |
THE AMERICAN JOURNAL OF FORENSIC MEDICINE AND PATHOLOGY
2001;22:139-149.
AUTHORS: Carlos Delmonte, M.D., Ph.D.; Vera Luiza Capelozzi, M.D., Ph.D.
From the Forensic Institute of Medicine (C.D.) and Department of Pathology (V.L.C.),
University of São Paulo School of Medicine, São Paulo, Brazil.
CITATION: Delmonte C, Capelozzi VL; Morphologic determinants of asphyxia in lungs: a semiquantitative study in forensic autopsies. Am J Forensic Med Pathol (United States), Jun 2001, 22(2) p139-49.

Asphyxia is a name given to different kinds of lesions that can produce similar histologic findings. Thus, because of the varied nature of the different kinds of lesions, as well as the incidence of similar qualitative histologic findings with different causes, the aim of this work was to study special kinds of injuries with particular subsequent impairment. These include some diagnostic problems of sudden death of natural causes, including aspiration, suffocation, drowning, and strangulation. Ranking was made of 167 victims based on the diagnosis as having: aspiration (n = 35), suffocation (n = 88), drowning (n = 27), and strangulation (n = 17). Stepwise discriminant analysis of the resulting data showed that lung necropsies from victims of these four events could be distinguished from one another. Statistical differences among the four groups were observed for eight morphologic parameters. A robust discriminant function permitted an adequate classification of the four groups of disease in 85.03% of the cases. Lung autopsies with congestion, septal hemorrhage, and foreign body showed a specificity of 100% for victims of aspiration, whereas ductal overinsufflation, interstitial edema, and bronchiolar constriction showed a specificity of 81.8% in victims of suffocation. Intraalveolar edema and dilatation of the alveolar spaces with secondary compression of the septal capillaries characterized drowning. Victims of strangulation showed a strong alveolar hemorrhage, with alveolar collapse and overinsufflation, associated with bronchiolar dilatation. It is concluded that semiquantitative analysis of lung autopsies might be a useful supplementary histologic criterion to support the diagnosis of asphyxia.
Key Words: Asphyxia; Semiquantitative analysis; Pathology

In biologic systems, the extent and type of pathologic and toxicologic findings can often be correlated with the specific circumstances of the fatal event. While such correlations are never perfect, their use in forensic scientific investigations forms an important component of the experienced investigator's repertoire. In addition, enlightened interpretation of postmortem findings may assist in elucidating the circumstances of death when they either are unresolved or later are found to have been confabulated.
Deaths resulting from violent asphyxia demonstrate a relative scarcity of histopathologic findings.(1) Nevertheless, it is important to document the pathologic changes in such cases to exclude other forms of trauma or other modes of death that may denote murder made to appear as suicide or natural death.
In addition, it appears to us that subtle differences in the particular constellation of histopathologic changes within the asphyxia death category may reflect how death occurred, through a gradation and quantitation of pathophysiologic alterations based on both the rapidity of compromise of the respiratory tract and its degree of completeness.
Asphyxia is a name given to different kinds of lesions that can produce similar histopathologic findings.(2-8)
Thus, because of the varied nature of the different kinds of lesions, as well as the incidence of similar qualitative histologic findings with different causes, the aim of this work was to determine semiquantitative morphologic parameters in previous demographic and circumstantial data in 167 consecutive asphyxia deaths by aspiration, suffocation, drowning, and strangulation investigated by the Forensic Institute of Medicine in São Paulo.
METHODS
Demographic and Situational Data
The deaths included in this study consisted of 200 cases consecutively investigated from 1996 to 1998 by the Forensic Institute of Medicine. A complete postmortem examination was conducted in each case, either personally performed or individually supervised by the main author. Those few cases that demonstrated putrefactive changes so severe that adequate interpretation of postmortem findings was compromised were excluded from the study. Before the project was begun, a data protocol was prepared that included certain personal, demographic, circumstantial, and pathologic information. The prosecutor in each case completed the protocol.
Regarding definition of terms, characterization of cases was made according to a previous consensus that asphyxia in a forensic context is mechanical asphyxia, rather then some of the internal conditions, which are more likely to arise as a result of natural disease or toxic conditions. Several different names have been used to describe the various types of asphyxia. Because some of them are confusing or inexact, they were characterized as follows(4,5):
Aspiration: blockage of the airways by a foreign body. Suffocation: failure of blood oxygenation caused by entrapment or environmental suffocation, smothering, choking, mechanical asphyxia, or mechanical asphyxia combined with smothering and suffocating gases. Drowning: death occurring after immersion in water. Strangulation: a form of compression of the neck for hanging and ligature or manual strangulation.
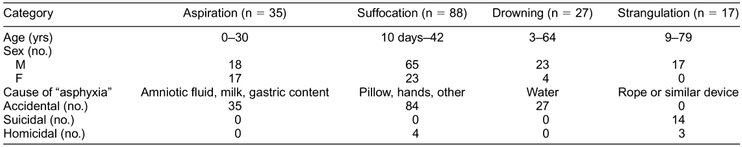
The cases were divided into four groups: group I, aspiration (n = 35); group II, suffocation (n = 88); group III, drowning (n = 27); and group IV, strangulation (n = 17). The diagnosis was based on legal expertise. In each case, data concerning the victim's age and sex, as well as the circumstances of death, are reported.(Table 1)


Technical Procedures to Obtain the Lungs
The necroscopic examination was done according to the routine procedure established by the Thanatology and Forensic Pathology Department of the Legal Medicine Institute of São Paulo. Briefly, the thoracotomy was done in the anterior medial line by sternopubic incision. The heart was removed by transection of the aortic and pulmonary vessels and the vena cava and pulmonary veins. The lungs were obtained by transection of the trachea 5 cm above the carina. After that, they were cut into 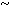 1-cm parasagittal slices, carefully examined for the presence of gross abnormalities, and fixed with 15% formalin solution for 24 hours. To avoid suspicion of respiratory hazards other than asphyxia, 33 of the 200 lungs collected were excluded because macroscopic and microscopic examinations of fixed lung slices indicated severe chronic bronchitis and/or emphysema and were obtained from older patients who were smokers (median age 54 years). Two or three sections of distal parenchyma (right middle lobe) were embedded in paraffin and processed according to conventional histologic procedures for optical microscopy. In fact, rather than the entire lung, the middle lobe was chosen because it is prone to have minor degrees of postmortem blood stagnation.
1-cm parasagittal slices, carefully examined for the presence of gross abnormalities, and fixed with 15% formalin solution for 24 hours. To avoid suspicion of respiratory hazards other than asphyxia, 33 of the 200 lungs collected were excluded because macroscopic and microscopic examinations of fixed lung slices indicated severe chronic bronchitis and/or emphysema and were obtained from older patients who were smokers (median age 54 years). Two or three sections of distal parenchyma (right middle lobe) were embedded in paraffin and processed according to conventional histologic procedures for optical microscopy. In fact, rather than the entire lung, the middle lobe was chosen because it is prone to have minor degrees of postmortem blood stagnation.
Slides 5 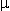 m thick were taken and stained with hematoxylin and eosin. All slides were coded, randomized, and then evaluated by a single observer who did not have access to the code.
m thick were taken and stained with hematoxylin and eosin. All slides were coded, randomized, and then evaluated by a single observer who did not have access to the code.
Morphologic Study
Qualitative Analysis
The histologic sections obtained for each case were first examined qualitatively to determine pulmonary architecture alterations and histopathologic lesions. In this way, the pulmonary tissue was divided into two histoanatomic compartments: the lobular compartment, represented by the membranous bronchioles, and the acinar compartment, represented by the respiratory bronchioles and the adjacent alveolar tissue (ducts, sacs, and alveoli). In all cases, histologic analysis of the pulmonary architecture showed the following alterations:
Alveolar tissue collapse was characterized at low magnification by collapse of the alveolar framework, involving alveolar sacs and ducts leading to overlap of the alveolar septa and reduction of the space for gas exchange. Figure 1 shows the histopathologic picture after the alveolar collapse.

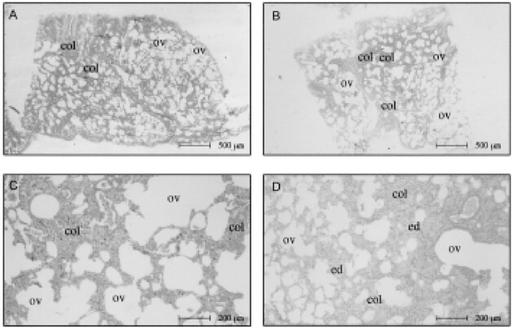
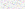 FIG. 1. Lung parenchyma in asphyxia by suffocation.(A- D) Alternating zones of ductal overinsufflation (ov) and alveolar collapse (col) of the lung parenchyma in suffocation cases.(B,D) Characteristic interstitial edema (ed).
FIG. 1. Lung parenchyma in asphyxia by suffocation.(A- D) Alternating zones of ductal overinsufflation (ov) and alveolar collapse (col) of the lung parenchyma in suffocation cases.(B,D) Characteristic interstitial edema (ed).

Alveolar tissue overinsufflation was revealed at low magnification by heterogeneous enlargement of the ducts, sacs, and alveoli, contrasting with the adjacent collapsed areas. Figure 1 and Figure 2A through F show the spatial arrangement of the alveolar tissue after overinsufflation.

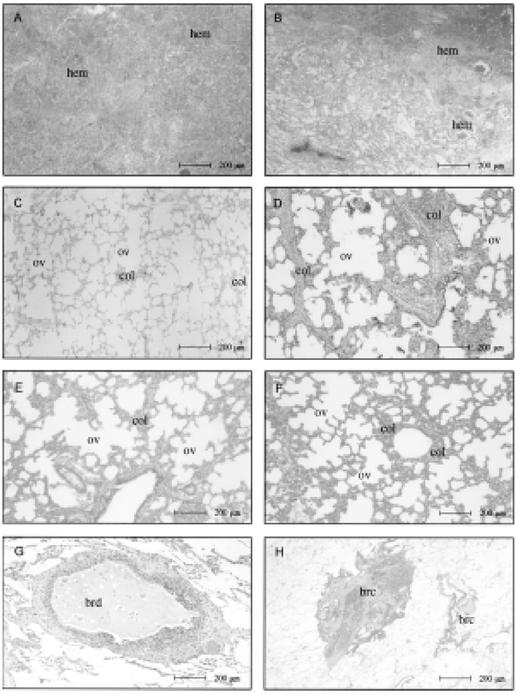
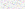 FIG. 2. Lung parenchyma in strangulation (suicidal hanging and homicidal ligature strangulation).(A,B) Intraalveolar hemorrhage (hem).(C-F) Alternating areas of alveolar collapse (col) and overinsufflation (ov).(G,H) Zones of bronchiolar constriction (brc) and dilatation (brd), a characteristic morphologic picture in this group.
FIG. 2. Lung parenchyma in strangulation (suicidal hanging and homicidal ligature strangulation).(A,B) Intraalveolar hemorrhage (hem).(C-F) Alternating areas of alveolar collapse (col) and overinsufflation (ov).(G,H) Zones of bronchiolar constriction (brc) and dilatation (brd), a characteristic morphologic picture in this group.

Bronchiolar constriction was characterized by luminal reduction of the membranous and respiratory bronchioles, enhanced by the virtual increase in the thickness of muscle layer and epithelium corrugation. Figure 2H shows a typical case. Bronchiolar dilatation was shown by the increase of internal diameter of the membranous and respiratory bronchioles and consequent thinning wall.(Fig. 2G)
Then, each histoanatomic compartment was examined for histopathologic lesions according to the basic principles of general pathology: cell injury, inflammation/repair, and circulatory alterations. Thus, the histopathologic lesions in each case examined constituted a morphologic substrate of general pathologic changes related to the circulatory alterations, specifically edema, intraalveolar deposition of proteic and amorphous material, passive congestion, and hemorrhage, characterized as follow:
Edema was recorded when proteic, amorphous, eosinophilic material was present inside the alveolar septa.(Fig. 1D) Intraalveolar deposition of proteic and amorphous material was observed in two situations. In the first, the amorphous material filled the alveolar spaces in a uniform and homogeneous pattern similar to that of hydrostatic edema, characterizing the near-edema seen in Figure 3A and B. In the second, the presence of a heterogeneous and lightly basophilic material (Fig. 4C,D) filling the alveolar spaces characterized the presence of a foreign body (milk, amniotic fluid).

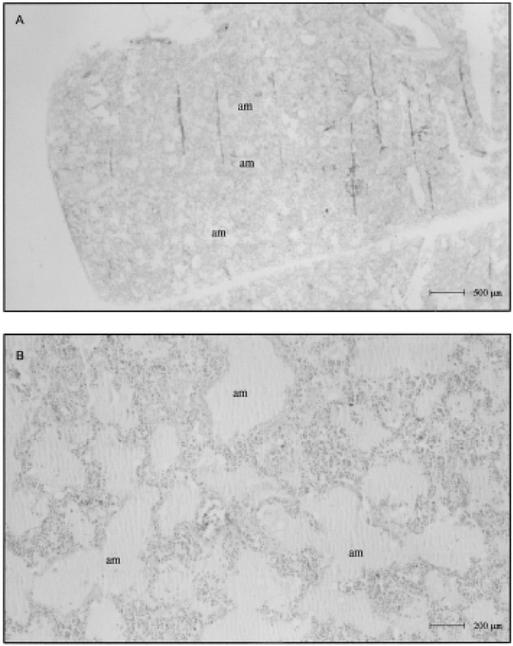
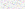 FIG. 3. Lung parenchyma in drowning cases.(A) Panoramic view of intraalveolar deposition of proteic and amorphous material (am) (B) High-magnification view showing acute dilatation of the alveoli with extension, elongation, and thinning of the septa and compression of the alveolar capillaries by a prominent intraalveolar reddish liquid similar to edema (am).
FIG. 3. Lung parenchyma in drowning cases.(A) Panoramic view of intraalveolar deposition of proteic and amorphous material (am) (B) High-magnification view showing acute dilatation of the alveoli with extension, elongation, and thinning of the septa and compression of the alveolar capillaries by a prominent intraalveolar reddish liquid similar to edema (am).

 FIG. 4. Lung parenchyma in aspiration cases.(A-D) Characteristic histologic appearance of congestion areas (cong) and engorged capillaries protruding into the alveolar lumen (arrows), as well as the foreign body occupying the bronchiolar and alveolar lumen (arrowheads).(E,F) Septal hemorrhage (double arrows), a typical morphologic reaction finding in this.
FIG. 4. Lung parenchyma in aspiration cases.(A-D) Characteristic histologic appearance of congestion areas (cong) and engorged capillaries protruding into the alveolar lumen (arrows), as well as the foreign body occupying the bronchiolar and alveolar lumen (arrowheads).(E,F) Septal hemorrhage (double arrows), a typical morphologic reaction finding in this.

Passive congestion was the anatomic substrate of the venous stasis in the pulmonary microcirculation. It was characterized by capillary distention, in which dilatation and sinuosity caused a spatial distortion of the alveoli, thereby being responsible for the increased color features observed in the tissue at optical microscopy.(Fig. 4A,B) Hemorrhage was characterized by the presence of blood inside the alveolar spaces (Fig. 2A,B) or along the alveolar septa (Fig. 4E,F); in both situations, the background structures were blurred.
Quantitative Analysis
Because the qualitative study revealed common morphologic parameters for the four groups of asphyxia, the next procedure included the semiquantitative analysis of the previous parameters established, as follows:
Parameters related to pulmonary architecture
Alveolar tissue collapse
Alveolar tissue overinsufflation (alveolus and ducts)
Bronchiolar constriction (membranous and respiratory bronchioles)
Bronchiolar dilatation (membranous and respiratory bronchioles)
Parameters related to histopathologic lesions
Interstitial edema
Intraalveolar deposition of proteic
and amorphous material (near-edema, foreign body)
Passive congestion
Hemorrhage (intraalveolar and interstitial)
These parameters were semiquantified by a histopathologic score according to the extent and severity of the histopathologic lesions present in total tissue, examined as follows:
0: Absence of lesion
1: Presence of lesions in 1% to 25%
2: Presence of lesions in 26% to 50%
3: Presence of lesions in 51% to 75%
4: Presence of lesions in 76% to 100%
Statistical Analysis
Discriminant analysis was used to obtain a statistical classification of the four groups. This method permits finding the linear/additive combination of variables that gives the clearest separation of individuals into different groups. It includes identification of the variables that contribute significantly to discrimination. The criterion for inclusion of a variable was an F value of  3.0, roughly corresponding to P = .04. Further independent combinations of the same variables were also calculated. Classification involves determining a separate prediction equation corresponding to each group that gives the probability of belonging to that group. A stepwise procedure was used to select the variables relevant to distinguishing the groups. Because the discriminant power would be optimistic when assessed on the same data used to derive the functions, a jackknife (one-out) procedure was included in the results. In short, this procedure withdraws one victim (victim 1, for instance) from the analysis, then the model is reestimated excluding that victim. Afterward, the excluded victim is classified according to the new model, and his or her actual classification is compared with that predicted. Next, victim 1 is again included in the analysis and victim 2 is withdrawn, according to the same procedure, until calculations are completed for all victims included in the study. All statistical procedures were done by use of the SPPS (version 6.0) statistical package(9), and the level of significance was 0.5%.
3.0, roughly corresponding to P = .04. Further independent combinations of the same variables were also calculated. Classification involves determining a separate prediction equation corresponding to each group that gives the probability of belonging to that group. A stepwise procedure was used to select the variables relevant to distinguishing the groups. Because the discriminant power would be optimistic when assessed on the same data used to derive the functions, a jackknife (one-out) procedure was included in the results. In short, this procedure withdraws one victim (victim 1, for instance) from the analysis, then the model is reestimated excluding that victim. Afterward, the excluded victim is classified according to the new model, and his or her actual classification is compared with that predicted. Next, victim 1 is again included in the analysis and victim 2 is withdrawn, according to the same procedure, until calculations are completed for all victims included in the study. All statistical procedures were done by use of the SPPS (version 6.0) statistical package(9), and the level of significance was 0.5%.
RESULTS
Individual Morphometric Measurements
Figure 5, Figure 6 and Figure 7 show the morphometric data for the 167 serial autopsies. Alveolar hemorrhage (Fig. 7A), congestion (Fig. 5A), alveolar collapse (Fig. 7B), alveolar overinsufflation (Fig. 7C), bronchiolar constriction (Fig. 6B), and bronchiolar dilatation (Fig. 8D) were higher in strangulation, whereas septal hemorrhage (Fig. 5C) and foreign body (Fig. 5B) were more frequent in aspiration. Intraalveolar edema (Fig. 6C) and ductal overinsufflation (Fig. 6A) with interstitial edema (Fig. 6D) characterized drowning and suffocation, respectively.

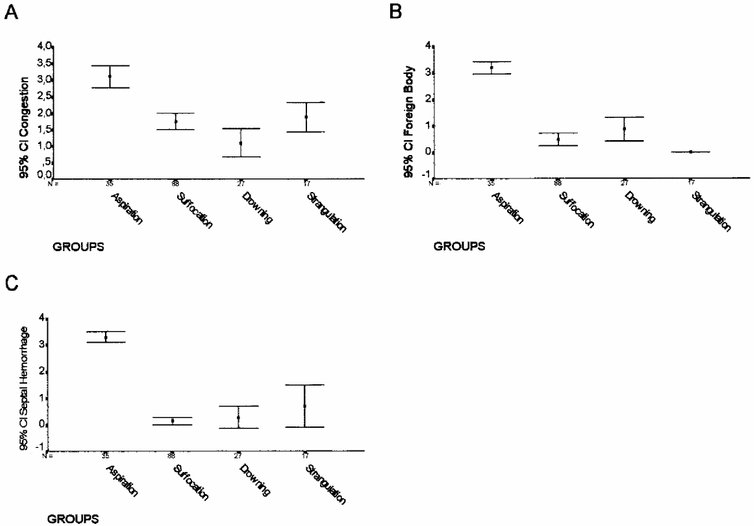
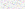 FIG. 5. Extension area (%) of congestion (A), foreign body (B), and septal hemorrhage
FIG. 5. Extension area (%) of congestion (A), foreign body (B), and septal hemorrhage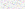 (C) in the four groups.
(C) in the four groups.

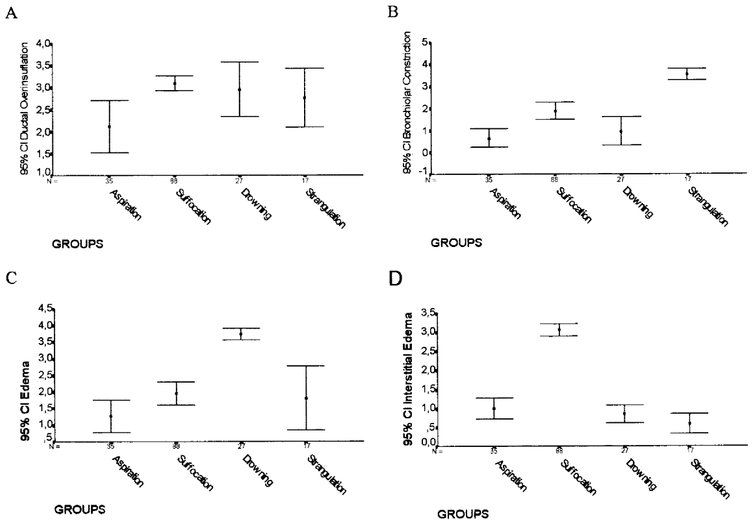
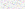 FIG. 6. Extension areas (%) of ductal overinsufflation (A), bronchiolar constriction
FIG. 6. Extension areas (%) of ductal overinsufflation (A), bronchiolar constriction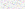 (B), intraalveolar edema (C), and interstitial edema (D) in the four groups.
(B), intraalveolar edema (C), and interstitial edema (D) in the four groups.

 FIG. 7. Extension areas (%) of alveolar hemorrhage (A), alveolar collapse
FIG. 7. Extension areas (%) of alveolar hemorrhage (A), alveolar collapse (B), alveolar overinsufflation (C), and bronchiolar dilatation (D) in the four groups.
(B), alveolar overinsufflation (C), and bronchiolar dilatation (D) in the four groups.

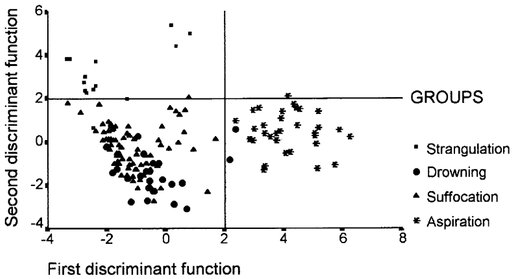
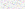 FIG. 8. The results of stepwise discriminant analysis.
FIG. 8. The results of stepwise discriminant analysis.

Statistical Analysis
Different combinations of the morphometric data by discriminant analysis selected eight variables capable of distinguishing the groups. These are given here, with P values relating to removal from the model: septal hemorrhage (P < .001), foreign body (P < 0.001), alveolar hemorrhage (P < .001), bronchiolar dilatation (P < .001), edema (P < .001), bronchiolar constriction (P < .001), ductal overinsufflation (P < .001), and alveolar collapse (P < .001). These relevant variables were used to construct the model shown in Figure 8, where a plot of the values of the first two linear discriminant functions for each individual nearly shows the separation achieved between the groups. The solution generated by discriminant analysis allowed us to distinguish four distinct patterns of asphyxia: aspiration, suffocation, drowning, and strangulation.
Aspiration
In this pattern, extension of the area occupied by congestion (Fig. 4A-D) and septal hemorrhage (Fig. 4E,F) was significantly higher in aspiration. In addition, and as expected, in these groups semiquantitative analysis of a foreign body (Fig. 4C,D) was statistically higher in aspiration than in the other groups. Congestion and hemorrhage allowed a distinctive histologic pattern to be highly associated with a diagnosis of aspiration, in which a septal hemorrhage was the typical morphologic reaction.(Fig. 4E,F) In addition to this picture of hemorrhage, there was considerable foreign body occupation of the lumen of the bronchioles and alveoli.(Fig. 4C,D) Thirty aspiration victims (100%) were properly classified as having aspiration.(Table 2)


Suffocation
In the second pattern of asphyxia, the extension of the area occupied by ductal overinsufflation (Fig. 1) and interstitial edema (Fig. 1B,C) were statistically more frequent in suffocation than in drowning or strangulation. Here, there was no interstitial edema, and the ductal overinsufflation was present in a minor degree. In suffocation cases, equally present but in a minor degree were the alternating zones of alveolar overinsufflation and collapse. Bronchiolar constriction was present in both suffocation and strangulation, whereas intraalveolar edema was a common parameter between suffocation and drowning cases. However, ductal overinsufflation in the form of acute substantial emphysema was characteristically present in most cases of death by suffocation. Seventy-two (81.8%) of the 88 victims of suffocation elsewhere were properly classified in the group in which the cause of the death was associated with suffocation. Sixteen patients of this group were misclassified (Table 2) and fell into the group of drowning by strangulation.
Drowning
In the third pattern of asphyxia, extension of the area occupied by intraalveolar deposition of proteic and amorphous material (Fig. 3A,B) was the semiquantitative parameter statistically associated with drowning. The other parameters, although still pres-ent, were not as statistically significant in drowning as the edema was.
Strangulation
The extension of the area occupied by alveolar hemorrhage (Fig. 2A,B) was significantly higher in lungs associated with strangulation. Equally significant was the association of alveolar collapse (Fig. 2B,C), alveolar overinsufflation (Fig. 2E,F), and alternating zones of bronchiolar constriction and bronchiolar dilatation (Fig. 2G,H). This particular morphologic picture thus characterized the lung involvement in the fourth group of asphyxia.
DISCUSSION
Asphyxia can be defined as the injuries caused by oxygen deficiency (hypoxia) that involve all conditions and sequelae caused by impairment or interruption of the oxygen supply or utilization in the tissues. Conversely, the term suffocation in forensic medical usage is restricted, for practical purposes, to cases in which environmental suffocation (inadequate oxygen in the atmosphere due to environmental conditions), smothering (due to mechanical obstruction of the nose and mouth), choking (due to blockage of the internal airways), and mechanical (due to pressure on the chest). In forensic practice, the recognition of death through asphyxiation can present problems if there are no indicative external or internal injuries or obstruction of the respiratory tract, and the conditions of death are not known in detail. In addition, the known macroscopic and histologic signs of general damage through hypoxia, e.g., edema, hemorrhage, pulmonary emphysema, passive congestion, and degenerative cellular changes, are usually diverse and are not conclusive as individual findings.(10-11) Similar findings can also arise through injuries leading to impairment of the circulation, or ischemia, thereby reducing or interrupting the tissue oxygen supplies. The extent of terminal hypoxia is also relevant to the postmortem detectable changes.(12) Therefore, the differential diagnosis of asphyxia has been limited by the qualitative similarity of the gross and histopathologic features in pulmonary involvement. In the absence of specific criteria, interpretation of a morphologic picture in asphyxia challenges the pathologist mainly when the same morphologic parameter is a common feature among the cases.
In this work, qualitative study revealed common morphologic parameters for the four groups of asphyxia. In all cases histologically analyzed, the pulmonary architecture showed variables in the degree of alveolar tissue collapse, overinsufflation, bronchiolar constriction, and dilatation. Equally common among the four groups were the histopathologic lesions: edema, intraalveolar deposition of proteic and amorphous material, passive congestion, and hemorrhage. However, we have shown that by means of a simple and quick semiquantitative method, with no additional cost to the Forensic Institute, it is possible to further characterize four specific groups of asphyxia and to suggest a specific diagnosis with reasonable accuracy. The method also allowed obtaining complementary parameters to refuse natural causes of death. Analysis of the results allowed us to identify four distinct patterns of histologic lung involvement.
As aspiration lung was characterized by gastric content or amniotic fluid, partially or totally filling the bronchiolar lumen and the alveolar spaces, thus characterizing the foreign material aspirated. As a consequence, occlusion of the small airways, mainly the membranous and respiratory bronchioles, was a typical morphologic finding. This pattern of bronchiolar involvement was not uniform along the small airways and was in contrast to areas where bronchiolar constriction was also evident. Thus, the simultaneous occurrence of acute and vicarious emphysema, characterized by alternating areas of overinsufflated and collapsed alveoli, was evident. This morphologic disarrangement of the bronchiolar and alveolar architecture determines changes in the ventilation/perfusion relationship leading to acute vascular congestion and engorged capillaries protruding into the alveolar space, making recognition of the alveolar boundaries particularly difficult. In addition, septal hemorrhage was also evident. In these cases, differential diagnosis between intrapulmonary hemorrhages of various causes, such as hemorrhagic pulmonary edema and hemorrhagic pulmonary infarctions or pneumonia, may be possible by complementary findings, such as bronchiolar constriction in cases of acute aspiration.
An acutely asphyxiated lung, characterized by acute substantial emphysema (ductal overinsufflation), was the typical morphologic reaction seen in the lungs of victims of suffocation. Pathology panel members who studied the prevalence of histologic lesions in sudden infant death syndrome (13) obtained similar findings. These findings are opposite those of Simonin and colleagues (7), who reported that in death caused by suffocation, the cause of death could not be determined by autopsy alone because there were no specific findings. Another differential parameter in our cases of suffocated lungs was interstitial edema secondary to hypoxia. These findings are supported by animal experiments with asphyxiated lung (10,14-15), where the more frequently occurring pulmonary edema following hypoxia was attributed to the physiologically greater permeability of the capillaries in the lungs under conditions of hypoxia.
In the third pattern of lung involvement, the use of semiquantitative information, besides improving the morphologic characterization of drowned lung, led to the identification of victims that should continue under diagnostic investigation. As shown in Table 2, the discriminant model misclassifies mainly the groups involving drowning and suffocation. In this work, fresh-water drowned lung was characterized by acute dilatation of the alveoli with extension, elongation, and thinning of the septa and compression of the alveolar capillaries by a prominent intraalveolar intense rose-colored liquid similar to edema. Reidbord and Spitz (16) and Spitz et al. (17) described similar findings in victims drowning in fresh water, and unlike the findings of Knight (5), these positive signs of drowning were not scanty and nonspecific. The histopathologic picture of intraalveolar deposition of proteic and amorphous material (near-edema) found in fresh-water drowned lung has strong support in experimental studies on rats involving active aspiration of watery liquids of various osmolarities.(18) The pathophysiologic mechanism involves general hyperhydration of the submicroscopic cell organelles, as mitochondria and endoplasmic reticulum, which in turn brings about swelling and the intracytoplasmic vesicular formation of pneumocytes and capillary endothelia.(10,18)
In the fourth group of asphyxia, strangulation, alternating areas of bronchiolar constriction and dilatation leading to alveolar collapse and overinsufflation, associated with a picture of alveolar hemorrhage, were the morphologic characteristics of lung involvement. These disturbed morphologic disarrangements of the bronchiolar and alveolar architecture determine changes in the circulation relationship leading to a particular reaction pattern-the alveolar hemorrhage-which enabled them to be distinguished from other forms of death. Accordingly, specific morphologic changes can be also expected in the parenchyma of the lungs in experimental cases of strangulation. In fact, rats and rabbits, with appropriate controls, showed a pronounced hemorrhagic syndrome, extending to all compartments of the lungs, to be a qualitatively and quantitatively prominent finding in strangulation. By means of semithin sections and electron microscopy, a distinct alveolar hemorrhage was also demonstrated that did not occur in other forms of death with short agony.(11) The pulmonary vascular system and pulmonary tissues thus constitute a target organ of strangulation agony.(11,18) The Hamburg working group around Brinkmann (11) has systematically studied the pathophysiologic processes that occur in the pulmonary vascular system and pulmonary tissue during strangulation. The object of these animal experiments and comparative pathologic studies in humans was the compilation of findings utilizing histopathologic staining methods and forensic-medical assessments. Further investigations concerning these problems involve the frequency of pulmonary hemorrhage in death by strangulation (19) and the question of acute emphysema in strangulation.(20)
We conclude that semiquantitative analysis of lungs at autopsy can be useful as a supplementary histologic criterion to support the diagnosis of asphyxia. The discriminant parameters obtained proper permitted classification in 85.03% of cases. This finding suggests that additional studies, including macroscopic characteristics, clinical data, and electron microscopy techniques, are probably required for better identification of asphyxia. However, because of its simplicity, efficiency, and low cost, the use of morphometric tools in the routine procedures should be encouraged in the analysis of death by asphyxia.

REFERENCES
Manuscript received June 1, 2000;
accepted June 8, 2000.
Supported by the Brazilian Funding Agencies FAPESP, CAPES, CNPq, and LIM-HCFMUSP.
Address correspondence and reprint requests to Vera Luiza Capelozzi, Departamento de Patologia, Faculdade de Medicina da USP, Av. Dr. Arnaldo 455, CEP 01246-009, São Paulo, SP., Brasil.

 Email Charly at: c-d-miller@neb.rr.com
Email Charly at: c-d-miller@neb.rr.com