CITATION:
Chan TC, Vilke GM, Neuman T, Clausen JL:
Restraint position and positional asphyxia.
Ann Emerg Med November 1997;30:578-586.
Although the "old" version of this article remains on this page,
in December, 2005, I created and posted a PDF file of this article!
If you're going to print it, I strongly suggest you print it from the PDF file:
Restraint Position and Positional Asphyxia
PLZ NOTE:
There are TWO PHOTOS posted on this webpage that are NOT in the ARTICLE that was published (the article in the PDF file).
Here they are:
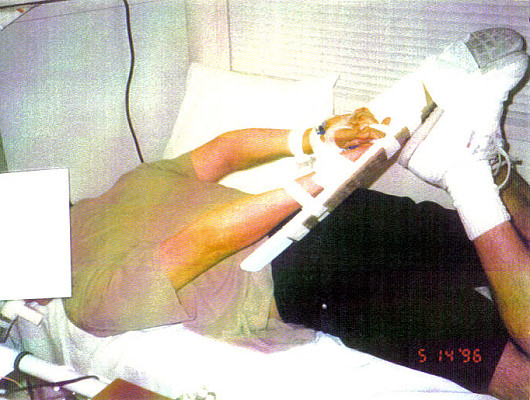
PHOTOGRAPHS of the study position that generated the DIAGRAM Chan et al commissioned for their November 1997 article's Figure 1.
(I have no idea why they didn't just |
 |
[Superfluous to all but the most obsessive reader, "Table" and "Figures 2 and 3" are posted at this page's END (just before the REFERENCEs). But, Table/Figures 2 & 3's descriptive captions are included within the article.]

Study Objective: To determine whether the "hobble" or "hogtie" restraint position results in clinically relevant respiratory dysfunction.
Methods: This was an experimental, crossover, controlled trial at a university-based pulmonary function laboratory involving 15 healthy men ages 18 through 40 years. Subjects were excluded for a positive urine toxicology screen, body mass index (BMI) greater than 30 kg/m2, or abnormal screening pulmonary function testing (PFT). Forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), and maximal voluntary ventilation (MVV) were obtained with subjects in the sitting, supine, prone, and restraint positions. After a 4-minute exercise period, subjects rested in the sitting position while pulse, oxygen saturation, and arterial blood gases were monitored. The subjects repeated the exercise, then were placed in the restraint position with similar monitoring.
Results: There was a small, statistically significant decline in the mean FVC (from 5.31±1.01 L [101%±10.5% of predicted] to 4.60±.84 L [88%±8.8% of predicted]), mean FEV1 (from 4.31±.53 L [103%±8.4%] to 3.70±.45 L [89%±7.7%]), and mean MVV (from 165.5±24.5 L/minute [111%±17.3%] to 131.1±20.7 L/minute [88% ±16.6%]), comparing sitting with restraint position (all, P<.001). There was no evidence of hypoxia (mean oxygen tension [PO2] less than 95 mm Hg or co-oximetry less than 96%) in either position. The mean carbon dioxide tension (PCO2) for both groups was not different after 15 minutes of rest in the sitting versus the restraint position. There was no significant difference in heart rate recovery or oxygen saturation as measured by co-oximetry and pulse oximetry.
Conclusion: In our study population of healthy subjects, the restraint position resulted in a restrictive pulmonary function pattern but did not result in clinically relevant changes in oxygenation or ventilation.

Recent attention has focused on the use of the "hogtie" or "hobble" restraint position and its possible role in the sudden deaths of individuals placed in this custody restraint position.(3-6) In this restraint, individuals are placed in the prone position with their wrists handcuffed or tied together behind their backs and their ankles bound together and secured to their wrists. It has been suggested that this position adversely affects a person's ability to breathe by interfering with chest wall and abdominal movements necessary for normal breathing.(7)
As a result, sudden, unexpected deaths in persons so restrained have been attributed to hypoventilatory respiratory failure from body position or "positional asphyxia."(3-6) This theory has been based primarily on the work of Reay et al,(7) who reported that healthy individuals placed in the restraint position after periods of exercise had prolonged recovery times for heart rate and oxygen saturation as measured by pulse oximetry.
We sought to investigate "positional asphyxia" and respiratory compromise in subjects held in the restraint position. A two-phase study was conducted in healthy subjects to assess ventilatory function and gas exchange. In the first phase, pulmonary function was measured with the subject in various body positions, including the restraint position, to determine whether any compromise in ventilatory mechanics occurred. In the second phase, oxygenation and ventilation were monitored by arterial blood gas measurements, heart rate monitoring, and pulmonary function testing (PFT) to determine whether any ventilatory compromise or alteration in gas exchange occurred when the subject was placed in the restraint position after exercise.
MATERIALS AND METHODS
The experimental study design and protocol were reviewed and approved by the Human Subjects Committee of the University of California, San Diego. Fifteen healthy male volunteers between the ages of 18 and 40 years were enrolled in the study. Informed consent was obtained from all individuals who agreed to participate in the study. Subjects who completed the study were financially compensated.
Exclusion criteria included any history of pulmonary disease (including asthma), cardiac disease, recreational drug use, or other significant illness or disability that would limit the ability to perform the exercise regimen required for the study. Individuals with a body mass index (BMI, defined as the weight in kilograms divided by the square of the height in meters) greater than 30 kg/m2 were also excluded.
Potential subjects underwent both a urine toxicologic screening and screening PFT before acceptance into the study. Urine specimens were collected and tested by means of toxicologic immunoassay for the presence of urinary metabolites for the following drugs: marijuana, cocaine, amphetamines, phencyclidine, benzodiazepines, and opiates. Individuals were excluded if the immunoassay detected any of these substances in the urine.
Initial screening PFT were performed with spirometry as outlined in the next paragraph. Peak expiratory flow rates and lung volumes were measured while potential subjects were in the sitting position. Forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) were measured while the subject was in the sitting position with feet flat on the floor and back against the chair. Spirometry was performed in accordance with the American Thoracic Society criteria, including reproducibility within 5% variability on three repeat measurements.(8) Abnormal PFT results (FVC and FEV1) were defined as measurements below the fifth percentile of the normal range (1.65 times the SD for Student's one-tailed t test) for each subject with a given height, age, and race.(9,10) Abnormal results were verified with repeated measurements, and subjects with confirmed FVC or FEV1 values lower than 80% of predicted were excluded.
In phase 1 of the study, subjects underwent PFT in the following positions: sitting, supine, prone, and restraint. In the sitting position, the subject sat in a chair with his feet flat on the floor and his back upright against the back of the chair. In the supine position, the subject lay flat on his back on a medical examination table with his arms at his sides and his legs in full extension with the feet together. In the prone position, the subject lay flat on his stomach on a medical examination table with his head turned to the side, his arms at his sides, and his legs in full extension with the feet together. In the restraint position, the subject lay on his stomach. To allow secure placement of a radial artery line, each wrist and hand were taped to an armboard, which was then taped to the sole of the ipsilateral shoe with the knee flexed behind the subject (Figure 1). The metacarpal-phalangeal joints of the hands were placed in line with the heels of the feet. This position closely approximates the restraint position noted in previous studies and case reports in the prehospital setting.(3,4,7) The subject's feet were then taped together and his head was turned to the side. The order of positions (supine, prone, or restrained) was randomized to prevent any potential influence of serial testing on the measurements obtained. The sitting position was used last in all cases to allow for comparison with the initial screening PFT results and to ensure that no significant change had occurred (because of repeated measurements) since the initial screening PFT values. Measurements obtained in each of the four positions included FVC, FEV1, FEV1/FVC% (calculated), and maximal voluntary ventilation (MVV). MVV was repeated twice for a duration of at least 6 seconds to ensure reproducibility.
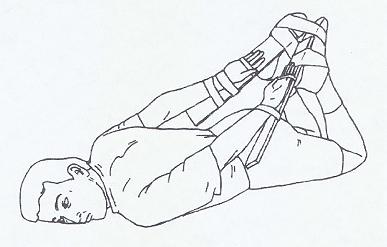 |
Diagram of restraint position. Subjects were placed in the prone position with hands and wrists taped to armboards behind the back and secured to the feet with knees flexed. Diagram by S Manitsas. |
PHOTOGRAPHS of the study position that generated the DIAGRAM Chan et al commissioned for their November 1997 article's Figure 1.
(I have no idea why they didn't just |
 |

In phase 2 of the study, subjects underwent two exercise periods and two rest periods. The first rest period occurred with the subject in the sitting position and the second in the restraint position; the order of positions was not randomized. During each rest period, serial arterial blood gas (ABG) measurements, pulse rate, and oxygen saturation by co-oximetry and pulse oximetry were recorded according to the protocol described in the following paragraphs. Additional PFT measurements were also performed during the rest periods, as described.
To allow for multiple blood gas samples, a 20-gauge radial arterial line was placed in one of the subject's wrists. Three-lead ECG monitor leads were placed for continuous heart rate monitoring. Transcutaneous oximetry was measured by pulse oximeter probes placed on the ear lobe ipsilateral to the side of the radial arterial line and on the index finger of the hand contralateral to the radial arterial line.
In the first exercise period, subjects exercised on a cycle ergometer at 175 W for 4 minutes. ABG samples were drawn immediately before the start of exercise and immediately after the end of exercise. Oxygen saturation by ear and finger probes and pulse rate by ECG tracing were recorded at the start of exercise, at 2 minutes into exercise, and at the end of exercise.
After the first exercise period the subject rested in the sitting position for 15 minutes. During this first rest period, blood samples for ABG analysis were obtained at 1.5 minutes and 15 minutes into rest. PFT was performed at 3 minutes into the first rest period. Oxygen saturation by both ear and finger probes and pulse rate by ECG tracing were recorded every 3 minutes during the first rest phase.
Once the heart rate had returned to less than 100 beats/ minute, the subject underwent a second exercise period, performing the same level of exercise as in the first period. Blood samples for ABG analysis were obtained before the start of exercise and immediately after the end of exercise. Oxygen saturation by both ear and finger probes and pulse rate by ECG tracing were recorded at the start of exercise, at 2 minutes into exercise, and at the end of exercise.
The subject was placed in the restraint position immediately after the second exercise period and remained in this position for 15 minutes. Blood samples for ABG analysis were obtained at 1.5 minutes and 15 minutes into the restraint period. PFT was performed at 3 minutes into the restraint period. Oxygen saturation by both ear and finger probes and pulse rate by ECG tracing were recorded every 3 minutes during this period. All ABG sampling and analyses were performed in a uniform manner, and results were verified by repeated testing of each sample on separate machines that were both internally and manually calibrated on a daily basis.
Raw PFT measurements were converted to percentages of predicted values (% predicted) for each subject to allow for normalization for age, height, and race.(9,10) Results are reported as mean±SD. For phase 1 of the study, one-way ANOVA for repeated measures, with position as the factor, and Student's t test were used to detect any statistically significant difference in the randomized PFT measurements. A probability value of less than .05 was considered statistically significant. For phase 2 of the study, two-way ANOVA for repeated measures, with position and time as the factors, and Student's t test were used to detect any statistically significant difference in PO2, co-oximetry, PCO2, and pulse rate between the two rest periods (ie, sitting versus restrained position). A probability value of less than .05 was considered statistically significant. We used a computerized statistical package for these analyses.
RESULTS
Two potential subjects were excluded for abnormal screening PFT measurements, and another individual was excluded for BMI greater than 30 kg/m2. None of our potential subjects had a urine toxicology screen result that was positive for recreational drug use.
The results of this study demonstrated significant changes in both static and dynamic pulmonary function testing with position and exercise. FVC was significantly decreased (P<.001) in the supine position (mean, 4.95±.93 L [94% ±9.9% of predicted]; range, 3.21 to 7.20 L), the prone position (4.92±.95 L [94%±10.2%]; range, 3.28 to 7.18 L), and the restraint position (4.60±.84 L [88%±8.8%]; range, 3.34 to 6.54 L), compared with the sitting position (5.31 ±1.01 L [101%±10.5%]; range, 3.65 to 7.90 L). FVC was also significantly less (P<.001) in the restraint position compared with the supine or the prone position, but the difference between the supine and prone positions was not significant. Exercise appeared to have no effect on the FVC in the sitting position (5.32±1.03 L [101%±10.5%]; range, 3.88 to 8.00 L; P>.30). However, in the restraint position, exercise caused a statistically significant increase in the FVC (4.74±.84 L [91%± 8.7%]; range, 3.47 to 6.89 L; P<.028). The Table reveals FVC results in terms of percentage of predicted value and the magnitude of change by position.
Table. Changes in static and dynamic pulmonary function tests in various positions. Percentages of predicted values were determined after normalization for height, age, and race in sitting position.(9,10) Probability values reflect significance compared with pre-exercise sitting values.
FEV1 decreased similarly to FVC with change in position. FEV1 was significantly less (P<.001) in the supine position (3.99±.54 L [95%±8.7%]; range, 2.81 to 4.79 L), the prone position (3.94±.52 L [94%±8.8%]; range, 2.83 to 4.63 L), and the restraint position (3.70±.45 L [89%±7.7%]; range, 2.90 to 4.44 L), compared with the sitting position (4.31± .53 L [103%±8.4%]; range, 3.19 to 4.95 L). The FEV1 was also less in the restraint position compared with the supine or the prone position (P<.001), but the difference between the supine and prone positions was not significant. Exercise appeared to increase the FEV1 in both the sitting position (4.47±.52 L [107%±9.1%]; range, 3.19 to 4.95 L; P<.001) and the restraint position (3.93±.83 L [94%±8.7%]; range, 3.17 to 4.51 L; P<.001). Because there were similar decreases in FEV1 and FVC, there were no significant changes in FEV1/FVC% (P>.15). The Table reveals FEV1 and FEV1FEV1/FVC% results in terms of percentage of predicted value and the magnitude of change by position.
MVV decreased in a statistically significant fashion (P<.001) from the sitting (165.5±24.5 L/minute [111%± 17.3%]; range, 128 to 210 L/minute), to the supine (151.5± 22.2 L/minute [101%±13.9%]; range, 105 to 185 L/minute), to the prone (143.5±20.0 L/minute [96%±14.8%]; range, 109 to 185 L/minute), to the restraint positions (131.1±20.7 L/minute [88%±16.6%]; range, 93 to 173 L/minute). The Table reveals MVV results in terms of percentage of predicted value and the magnitude of change by position.
This study also revealed significant changes in gas exchange with exercise in both the sitting and the restraint position. In the sitting position, gas exchange improved with exercise (P<.001). The PO2 increased from a baseline of 91.4±7.2 mm Hg (range, 81 to 105 mm Hg) to 108.7±8.0 mm Hg (range, 96 to 125 mm Hg) after 4 minutes of exercise. After 1.5 minutes of rest the PO2 was 122.7±5.8 mm Hg (range, 110 to 132 mm Hg); after 15 minutes of rest it had fallen back toward baseline and was 102.4±8.7 mm Hg (range, 84 to 115 mm Hg). The PCO2 also changed significantly with exercise (P<.001). From a baseline of 38.5±2.8 mm Hg (range, 31 to 41 mm Hg), PCO2 fell to 34.5±3.9 mm Hg (range, 29 to 41 mm Hg) after 4 minutes of exercise. The PCO2 was 31.3±2.6 mm Hg (range, 28 to 36 mm Hg) after 1.5 minutes of rest; after 15 minutes of rest the PCO2 remained lower than baseline at 32.9±2.7 mm Hg (range, 28 to 37 mm Hg). Because the changes in both PCO2 and PO2 were statistically significant, the change in the alveolar-arterial oxygen gradient across time was also significant (P<.001).
Immediately after exercise, just before the subject was placed in the restraint position, the PO2 had again improved (P<.012), from a baseline of 102.4±8.7 mm Hg (range, 84 to 115 mm Hg) to 109.8±9.3 mm Hg (range, 93 to 128 mm Hg) after 4 minutes of exercise. As the subjects were being placed in the restraint position (1.5 minutes after the end of exercise) the PO2 increased further to 114.0±7.6 mm Hg (range, 103 to 128 mm Hg), and after 15 minutes in the restraint position the PO2 was 99.1±9.3 mm Hg (range, 86 to 120 mm Hg). Likewise, the PCO2 fell (P<.001) with exercise, from an initial 32.9±2.7 mm Hg (range, 28 to 37 mm Hg) to 30.7±3.8 mm Hg (range, 24 to 37 mm Hg) after 4 minutes of exercise, just before the subject was placed in the restraint position. As the subjects were being placed in the restraint position the PCO2 was 31.0±3.4 mm Hg (range, 26 to 37 mm Hg); after 15 minutes in this position the PCO2 remained lower than baseline at 32.7±3.2 mm Hg (range, 26 to 38 mm Hg).
Comparing the ABG results in the sitting position to the results as the subjects were being placed in the restraint position (ie, 1.5 minutes into rest in the sitting position versus 1.5 minutes into the restraint position), the PO2 increased to a smaller value with restraint than with sitting (114.0±7.6 mm Hg versus 122.7±5.8 mm Hg; P<.01), whereas the PCO2 was the same in both groups (31.3±2.6 mm Hg versus 31.0±3.4 mm Hg; P>.28). Comparison of ABG results after 15 minutes in the sitting position with those after 15 minutes in the restraint position revealed no significant differences in either the PO2 (102.5±8.7 mm Hg versus 99.1 ±9.3 mm Hg; P>.234) or the PCO2 (32.9±2.7 mm Hg versus 32.7±3.2 mm Hg; P>.30).
Changes in heart rate also occurred with exercise, with a maximum heart rate of 164±18.9 beats/minute (range, 218 to 180) at the beginning of the sitting rest period and 174±15.3 beats/minute (range, 146 to 198) at the beginning of the restraint rest period. Throughout the two rest periods, there was no statistically significant difference in mean heart rate recovery (Figure 2).
Figure 2. Heart rate recovery during resting periods: sitting versus restraint positions. The X-axis indicates time into each rest period (in minutes) after cessation of exercise. The Y-axis indicates mean heart rate in beats/minute for the 15 subjects. Standard deviation of the mean is shown by error bars.
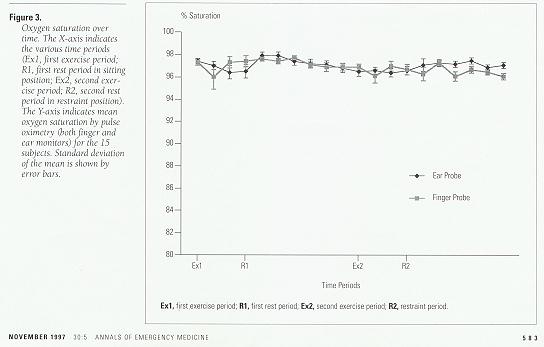
Examination of oxygen saturation as measured by co-oximetry revealed only minor, nonsignificant increases over the initial baseline. Comparison between oxygen saturation values measured by co-oximetry and by pulse oximetry also revealed no statistically significant differences for any time period (Figure 3).
Figure 3. Oxygen saturation over time. The X-axis indicates the various time periods (Ex1, first exercise period; R1, first rest period in sitting position; Ex2, second exercise period; R2, second rest period in restraint position). The Y-axis indicates mean oxygen saturation by pulse oximetry (both finger and ear monitors) for the 15 subjects. Standard deviation of the mean is shown by error bars.
DISCUSSION
The term "positional or mechanical asphyxia" has been used to explain the deaths of certain individuals. Bell et al(11) described 30 such cases of individuals whose bodies were found in positions that caused either external airway obstruction or inadequate ventilatory function. In all cases, there were no other significant life-ending pathologic findings and the deaths were attributed to "positional asphyxiation." Similar asphyxiation deaths have been described involving the use of vest, jacket, or posey restraints that were accidentally wrapped around the necks of nursing home and geriatric patients and resulted in strangulation.(12-15) There have been a few reports of ventilatory failure and asphyxiation caused by restraints that reportedly compressed the chest and abdomen to the point that mechanical ventilatory function was impaired.(16-18) Recently, attention has focused on the possible role of "positional asphyxia" in deaths that have occurred in persons restrained in the "hobble" or "hogtie" position by law enforcement or prehospital field personnel. This position places the individual prone with the wrists and ankles bound behind the back.(2-6) Reay et al(3) suggested that this position prevents adequate chest wall expansion and abdominal and diaphragmatic excursion for normal ventilatory function and breathing. They postulated that this inability to expand the thoracic cavity, and disadvantage in pulmonary mechanics, leads to hypoventilatory respiratory compromise, asphyxiation, and death.
Hirsh(18) argued that the employment of this restraint position constitutes use of a "potentially lethal force" and that such deaths should be classified as homicides. However, most of the reported cases have involved young men in a state of "excited" or "agitated delirium" as a result of intoxication from recreational drugs or psychiatric illness. In addition, these individuals had often suffered traumatic injuries before and during placement in the restraint position. Many have argued that these other factors (ie, intoxication, stress, and trauma), as opposed to asphyxiation strictly due to body positioning, played a greater role in causing these deaths.(19,20)
Reay et al(7) studied 10 healthy individuals and found measurable physiologic effects with application of the restraint position after exercise. They reported that prolonged times were required for recovery to baseline in the restraint position after exercise, both for heart rate (mean, .40 minutes longer) and for peripheral oxygen saturation measured by transcutaneous ear probe (mean, .33 minutes longer). Based on these findings, they suggested that positional asphyxia plays an important role in the deaths of persons placed in the restraint position.
A number of concerns exist with the work of Reay et al. First, there was no assessment of actual ventilatory function and respiratory mechanics in subjects placed in the restraint position. Second, although Reay et al reported a drop in oxygen saturation (to 85% to 90%) with exercise in their subjects, previous work has demonstrated improvements in arterial oxygenation with mild to moderate levels of exercise in healthy individuals.(21) Third, the preferred method for assessing arterial blood oxygenation remains ABG measurements. Oxygen saturation measurement by pulse oximetry has been shown to be a potentially inaccurate measure of arterial oxygenation, particularly during exercise.(22-25)
In this study, we assessed ventilatory function by PFT with subjects in various body positions. We found a restrictive pulmonary function pattern in healthy subjects placed in the restraint position, with small but significant decreases in the percentage of predicted FVC and FEV1. Associated with these drops in lung volumes was a corresponding 23% decrease in percentage of predicted MVV. There was no evidence of obstruction and no significant change in FEV1/ FVC% from baseline.
Given the fact that PFT measurements as low as 80% of predicted values are still considered clinically normal, these changes, although statistically significant, are not clinically relevant.(26) In reviewing the ranges of PFT data we obtained, none of our subjects had results for FVC and FEV1 lower than 80% of predicted in any of the positions, including the restraint position. The range of data for MVV did fall below 80% of predicted for certain outlier individuals in the restraint position, although similar decreases below 80% of predicted were also seen in the supine and prone positions.
In contrast to the studies of Reay et al, arterial oxygenation measurements were obtained in this study by both ABG sampling and transcutaneous finger and ear oxygen saturation probes. Based on arterial PO2 and co-oximetry, we found that oxygenation increases, rather than decreases, with exercise. This finding is consistent with previous, well-established work on exercise physiology.(21,27) In addition, despite our PFT findings, we found no evidence of hypoxia while subjects were in the restraint position after exercise. The improvements in oxygenation occurred in the face of a more vigorous exercise regimen than that described by Reay et al. Reay required subjects to exercise on a cross-country skiing machine only until their heart rates reached a maximum of 120 beats/minute. The subjects in this study exercised for 4 minutes continuously on an exercise bicycle, and the mean heart rate at the end of exercise was 169 beats/minute.
Equally important, despite decreases in MVV, there was no evidence of hypercapnia either during exercise or during rest in the restraint position. In fact, mean PCO2 levels decreased during exercise and remained lower than 40 mm Hg for as long as 15 minutes during the restraint rest position. Despite the restrictive pattern demonstrated by PFT, there was no evidence for ventilatory failure, significant hypoventilation, or asphyxiation as a result of body positioning while subjects were in the restraint position.
Based on these findings in healthy subjects, we suggest that factors other than body positioning are more important determinants for the sudden, unexpected deaths that occur in individuals who are placed in the restraint position. Recreational drug use (including sympathomimetic, hallucinogenic, and psychomotor stimulant drugs), physiological stress, hyperactivity, hyperthermia, catechol hyperstimulation, and trauma resulting from struggle may be more important factors in the deaths of these individuals.(19,20,28,29) Although restraints in general increase the psychological and physiologic stresses on the individual,(28,30) there is no evidence that body position while in the "hogtie" or "hobble" restraint position as a factor in and of itself causes hypoventilation or asphyxiation.
There are limitations to this study. First, we restricted subjects to healthy men between the ages of 18 and 40 years with a BMI less than 30 kg/m2; most cases reported in the literature involve this population. It is not known what effect positional restraint may have on women, the young, the elderly, or other individuals with underlying cardiopulmonary disease or disability. It is possible that extremely obese individuals with large abdominal girths and BMIs greater than 30 kg/m2 may be at greater risk for development of restrictive pulmonary function pattern as a result of abdominal compression from body position.
We specifically excluded potential subjects who had a positive result on urine toxicology screening for recreational drug use. As noted previously, many of the deaths of restrained individuals involved subjects who were intoxicated or under the influence of recreational drugs. Stimulants, such as cocaine and amphetamines, may increase oxygen demand and muscle fatigue, affecting overall respiratory function.
Although we randomized the order of positions for PFT, we did not randomize the sitting and restraint position rest phases after exercise in our study. All subjects rested in the sitting position first, after the initial exercise period, then exercised again and rested in the restraint position. We believe that 15 minutes of sitting rest should have been adequate time for values to return to baseline after 4 minutes of exercise. Any residual metabolic or respiratory derangements remaining after the first rest period would bias our study in favor of detecting significant abnormalities in the restraint position. In addition, we did not measure respiratory rate as an indicator of ventilatory status. We believe that the combination of MVV and serial PCO2 provides a more appropriate measure of ventilatory status than does respiratory rate.
This study did not attempt to duplicate exact field conditions under which restraint position deaths have occurred. Although many such deaths have occurred on gurney mattresses or cushioned car seats in the field, some deaths have occurred while persons were in the restraint position on the ground.(3) Deaths have also occurred on the floors of police cars, where the contoured surface may have increased abdominal compression.(3) In addition, these individuals may have been subject to forceful apprehension, during which pressure may have been exerted on their backs while they were in the restraint position. What effects these differences may have remain to be determined.
We kept our subjects in the restraint position for 15 minutes after the exercise period. We believe that this was adequate time to detect any physiologic or respiratory impairment in subjects. It is possible that had our subjects remained in the restraint position for a longer period we may have detected more significant alterations in respiratory physiology. However, most death of individuals in the restraint position have occurred after only a short period (often less than 10 minutes) in restraint,(3-6) and it has been suggested by others that this short duration of restraint positioning should not be fatal.(19)
We attempted to reproduce the physiologic effects of struggle by requiring our subjects to exercise for 4 minutes before being placed in the restraint position. It is unlikely that this period of exercise would simulate all the physiologic alterations that may occur with struggle and agitation. In addition, we did not reproduce the effects of trauma and psychological stress that often occur with apprehended individuals. However, the respiratory mechanics of our subjects (as evidenced by increased FVC and FEV1) improved with exercise.
It is possible that a combination of factors, including underlying medical condition, intoxication, agitation, delirium, and struggle as well as body position, may result in respiratory compromise that would not be detected by our study. We sought to examine only the role of body position as a factor affecting pulmonary function and respiratory physiology. Although we found that body position by itself does not result in significant respiratory compromise, further research is needed on the role of these other factors in the deaths of individuals placed in the restraint position.
In conclusion, although we found a small restrictive pulmonary function pattern by PFT parameters in subjects who were placed in the restraint position, we found no evidence of hypoxia or hypercapnia on serial ABG measurements. By itself, the restraint position was not associated with any clinically relevant changes in respiratory or ventilatory function in our study population of healthy individuals with preserved ventilatory reflexes and normal pulmonary physiology. There is no evidence to suggest that hypoventilatory respiratory failure or asphyxiation occurs as a direct result of body restraint position in healthy, awake, nonintoxicated individuals with normal cardiopulmonary function at baseline.
The authors thank Jeffrey Johnson, Carlos Lopez, and Paul Schragg for their assistance.

TABLE:
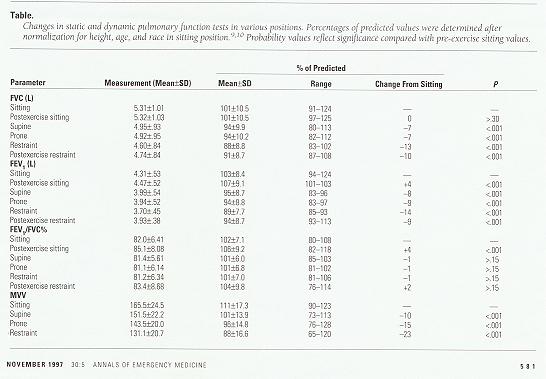
FIGURE 2:
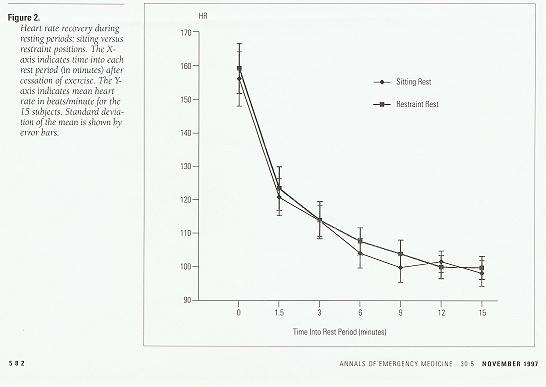
FIGURE 3:

REFERENCES
1. Reay DT, Eisele JW: Deaths from law enforcement neck holds. Am J Forensic Med Pathol 1982;3:253.
2. Luke JL, Reay DT: The perils of investigating and certifying deaths in police custody. Am J Forensic Med Pathol 1992;13:98.
3. Reay DT, Fligner CL, Stilwell AD, et al: Positional asphyxia during law enforcement transport. Am J Forensic Med Pathol 1992;13:90.
4. O'Halloran RL, Lewman LV: Restraint asphyxiation in excited delirium. Am J Forensic Med Pathol 1993;14:289.
5. Stratton SJ, Rogers C, Green K: Sudden death in individuals in hobble restraints during paramedic transport. Ann Emerg Med 1995;25:710.
6. Cowen AR: In a bind. A lawsuit forces LA medics and police to reexamine their policy on applying restraints. Emergency Medical Services 1995;24:54-8.
7. Reay DT, Howard JD, Fligner CL, Ward RJ: Effects of positional restraint on oxygen saturation and heart rate following exercise. Am J Forensic Med Pathol 1988;9:16.
8. American Thoracic Society: Standardization of spirometry: 1994 Update. Am J Respir Crit Care Med 1995;152:1107.
9. Morris JF: Spirometric standards for healthy non-smoking adults. Am Rev Respir Dis 1971;103:57.
10. Bass H: The flow volume loop: Normal standards and abnormalities in chronic obstructive pulmonary disease. Chest 1973;63:171.
11. Bell MD, Rao VJ, Wetli CV, et al: Positional asphyxiation in adults: A series of 30 cases from the Dade and Broward county Florida medical examiner offices from 1982 to 1990. Am J Forensic Med Pathol 1992;13:101.
12. Dube AH, Mitchell EK: Accidental strangulation from vest restraints. JAMA 1986;256:2725.
13. Katz L: Accidental strangulation from vest restraints. JAMA 1987;257:2032.
14. DiMaio VJM, Dana SE, Bux RC: Deaths caused by restraint vests. JAMA 1986;256:905.
15. Miles S: A case of death by physical restraint: New lessons from a photograph. J Am Geriatr Soc 1996;44:291.
16. Miles HS, Irvine P: Deaths caused by physical restraints. Gerontologist 1992;32:762.
17. Emson HE: Death in a restraint jacket from mechanical asphyxia. Can Med Assoc J 1994;151:985.
18. Hirsh CS: Restraint asphyxiation. Am J Forensic Med Pathol 1994;15:266.
19. Laposata EA: Positional asphyxia during law enforcement transport. Am J Forensic Med Pathol 1993;14:86.
20. Karch SB: Agitated delirium versus positional asphyxia. Ann Emerg Med 1995;26:760.
21. Wasserman K, Hansen JE, Sue DY, et al: Normal values, in Wasserman K, Hansen JE, Sue DY, et al (eds): Principles of Exercise Testing and Interpretation, ed 2. Philadelphia: Lea & Febiger, 1994:127-128.
22. Biebuyck JF: Pulse oximetry. Anesthesiology 1989;70:98.
23. Norton LH, Squires B, Craig NP, et al: Accuracy of pulse oximetry during exercise stress testing. Int J Sports Med 1992;13:523.
24. Hansen JE, Casaburi R: Validity of ear oximetry in clinical exercise testing. Chest 1987;91:333.
25. Brown DD, Knowlton RG, Sanjab PB, et al: Re-examination of the incidence of exercise-induced hypoxaemia in highly trained subjects. Br J Sports Med 1993;27:167.
26. Clausen JL: Pulmonary function testing, in Bordow RA, Moser KM (eds): Manual of Clinical Problems in Pulmonary Medicine, ed 4. Boston: Little, Brown, 1996:9-19.
27. Comroe JH (ed): Respiratory adjustments in health, in Physiology of Respiration, ed 2. Chicago: Yearbook Medical, 1975:234-241.
28. Mirchandani HG, Rorke LB, Sekula-Perlman A, et al: Cocaine-induced agitated delirium, forceful struggle, and minor head injury. Am J Forensic Med Pathol 1994;15:95.
29. Robinson BE, Sucholeiki R, Schocken DD: Sudden death and resisted mechanical restraint: A case report. J Am Geriatr Soc 1993;41:424.
30. Pudiak CM, Bozarth MA: Cocaine fatalities increased by restraint stress. Life Sci 1994;55:379.
From the Department of Emergency Medicine* and the Division of Pulmonary Medicine, Department of Medicine,‡ University of California San Diego Medical Center, San Diego, California.
Received for publication November 11, 1996.
Revision received March 24, 1997.
Accepted for publication May 8, 1997.
An abstract of this study was presented at the Society for Academic Emergency Medicine annual conference, Washington DC, May 1997.
Supported in part by grant No. 94-1974R from the County of San Diego and by grant MO1 RR0087 from the General Clinical Research Center, National Center for Research Resources, National Institutes of Health.
Reprint no. 47/1/85377
Address for reprints: Theodore Chan, MD, Department of Emergency Medicine,
University of California San Diego Medical Center,
200 West Arbor Drive #8676,
San Diego, California 92103
619-543-6463
Fax 619-543-3115
E-mail tcchan@ucsd.edu
Copyright (c) by the American College of Emergency Physicians.

A Published Letter to the Editor of Annals Of Emergency Medicine
by Forensic Pathologists Donald T. Reay and John D Howard, discussing their opinions
of Chan et al's Annals article, its research methods and conclusions.
This material originally posted here on August 1st, 1998.

A Comprehensive Review of Frequently Misinterpreted and
Misrepresented Restraint Research
This is the 2005 version of CHAS' OLD review of this article.
However, if you wish to read the OLD version, here it is:
Chan et al.'s 1997 Restraint Position Article is FLAWED
The OLD review was originally posted on August 5th, 1998.
Edited, updated, and re-posted in June, 2002.
And ultimately REPLACED by the 2005 review.

 Email Charly at: c-d-miller@neb.rr.com
Email Charly at: c-d-miller@neb.rr.com